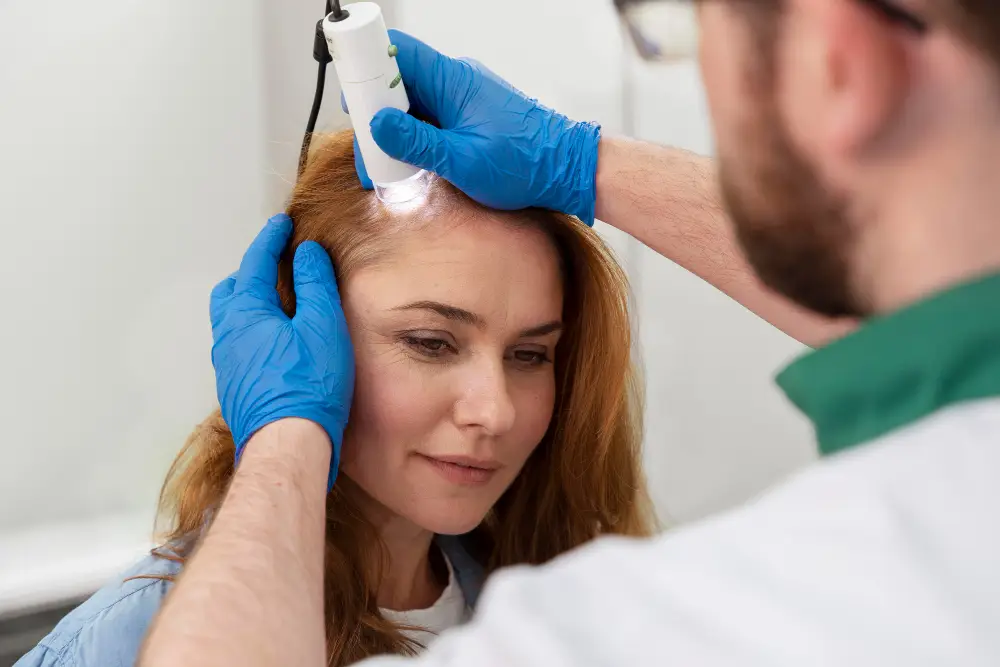
Ataxia Stem Cell Treatment: Understanding Your Options for Neural Repair
Key Takeaways
- Ataxia is a complex neurological condition impacting coordination, speech, and balance, with ongoing research into regenerative approaches like stem cell investigation.
- Traditional approaches focus on symptom management; emerging research in regenerative medicine, including stem cell studies, explores pathways for cerebellar regeneration and neural repair.
- For those exploring advanced options, understanding the scientific basis, potential research avenues, and patient journey support is critical.
- Pereira, Colombia, offers a supportive environment for individuals considering regenerative approaches, benefiting from a robust healthcare infrastructure and integrated patient advocacy.
- Making an informed decision requires thorough education, a confidential case review, and a clear understanding of what investigational treatments entail.
Introduction: Navigating the Complexities of Ataxia and Regenerative Pathways
A diagnosis of ataxia often brings with it a profound sense of uncertainty. This neurological condition, characterized by impaired coordination, can manifest in difficulties with balance, speech, swallowing, and fine motor control. The search for effective pathways often leads individuals and their families down many avenues, including exploring the cutting-edge field of regenerative medicine and investigational approaches like ataxia stem cell treatment.
At Regencord in Pereira, Colombia, we understand the deeply personal and often overwhelming journey that accompanies a diagnosis of ataxia. Our role is to provide comprehensive, ethical, and source-verified educational resources to empower you with the clarity needed to navigate these complex decisions. This article is designed not to offer medical advice or promise specific results, but to illuminate the current understanding of ataxia, the ongoing research in neural repair and cerebellar regeneration, and how a patient-centric framework can support your exploration of potential options, including those available in Pereira.
Many individuals living in countries like the USA face challenges in accessing a full spectrum of information regarding novel approaches, often feeling limited by conventional care models. We believe in transparency, patient advocacy, and presenting a balanced view of what investigational treatments entail, alongside the unique contextual advantages of considering care in a destination known for its high standards and patient support systems.
The Stakes: Critical Implications of Ataxia and the Search for Solutions

Ataxia impacts millions globally, and its progressive nature can significantly diminish quality of life. The condition results from damage to the cerebellum, the part of the brain responsible for coordinating voluntary movements, or to the nerve pathways that connect to it. This damage can stem from various causes, including genetic mutations, autoimmune reactions, infections, or exposure to certain toxins. The precise mechanisms behind cerebellar regeneration and how to promote it remain a significant area of research.
The World Health Organization (WHO) highlights neurological disorders, including ataxias, as leading causes of disability worldwide, underscoring the urgent need for advancements in understanding and managing these conditions. A key challenge is the progressive nature of many ataxias, where symptoms worsen over time, leading to increasing dependence and a diminished capacity for daily activities. This often prompts individuals to seek out all available avenues, including investigational therapies.
Research published in PubMed-indexed journals consistently explores various strategies for neural repair in ataxia, including pharmacological interventions, physical therapies, and, increasingly, regenerative approaches. The National Institutes of Health (NIH) actively funds research into the underlying causes of ataxia and potential therapeutic targets, emphasizing the long-term commitment of the scientific community to addressing this condition. While no definitive restorative treatment for ataxia is widely recognized, the field of regenerative medicine continues to explore possibilities for motor control recovery through understanding cellular mechanisms.
Source: World Health Organization (WHO), U.S. National Institutes of Health (NIH), PubMed-indexed peer-reviewed journals.
The Conventional Approach in the USA and the Quest for Innovation
In countries like the USA, the standard of care for ataxia primarily revolves around symptomatic management and supportive therapies. This includes physical therapy, occupational therapy, and speech therapy, all aimed at helping individuals maintain functionality and manage symptoms like poor coordination, tremors, and speech difficulties. Medications may be prescribed to address specific symptoms, such as muscle stiffness or dizziness, but these do not typically halt or reverse the progression of the underlying neurological damage.
The U.S. Food and Drug Administration (FDA) plays a crucial role in regulating medical treatments, ensuring their safety and efficacy through rigorous clinical trials. For many neurological conditions, including ataxia, the pace of developing new, disease-modifying therapies can be slow, leading many patients to feel that their options are limited. This can be a source of frustration and anxiety for individuals facing a progressive condition.
While the USA is at the forefront of medical research, the structured nature of its healthcare system, coupled with the slow regulatory approval process for novel therapies, can sometimes create barriers for patients seeking access to investigational treatments. This often prompts a global search for alternative pathways and research opportunities. The focus remains on robust clinical trials to establish the safety and potential benefit of any new treatment approach.
Source: U.S. Food and Drug Administration (FDA), U.S. National Institutes of Health (NIH).
The Pereira, Colombia Advantage: A Supportive Ecosystem for Regenerative Exploration
For those exploring advanced, investigational avenues for conditions like ataxia, including potential stem cell treatments, an international destination like Pereira, Colombia, offers compelling advantages beyond what might be conventionally available in countries like the USA.
Robust Healthcare Infrastructure and Regulation
Colombia has invested significantly in its healthcare infrastructure, with modern hospitals and clinics, particularly in major cities like Pereira. The Colombian Ministry of Health and INVIMA (Colombia’s National Food and Drug Surveillance Institute) are the primary governing entities, ensuring that medical facilities and procedures meet established national and international standards. INVIMA, similar to the FDA, regulates medical devices, pharmaceuticals, and biological products, providing an important layer of oversight for patient safety.
Governing Entities: Colombian Ministry of Health, INVIMA.
This regulatory environment supports a professional medical community, often with physicians trained internationally, providing a strong foundation for patients seeking care.
A Holistic Patient Journey Focused on Support
Navigating a diagnosis like ataxia often leaves individuals feeling isolated and without clear options in traditional healthcare systems. Our experience shows that the most critical early step is not treatment itself, but access to unbiased educational resources and a clear, guided pathway to informed decision-making, which is often missing. The patient journey in Pereira is designed to be comprehensive:
- Personalized Assessment: A confidential case review helps determine if investigational pathways are appropriate for an individual’s specific circumstances.
- Logistical Support: For those considering international care, particularly for complex conditions like ataxia, the logistical and emotional burden can be immense. What patients truly need is not just a medical procedure, but a comprehensive support ecosystem that addresses everything from travel and accommodation to cultural integration and post-procedure follow-up, transforming a daunting journey into a managed and supportive experience in a serene environment like Pereira.
- Empathetic Advocacy: The team at Regencord in Pereira, Colombia, acts as a dedicated patient advocate, guiding individuals through every step, from initial inquiry to post-procedure support. This personal approach aims to alleviate anxiety and foster trust.
Source: Synthesized contextual insights from deep experience in facilitating international medical care.
The Environment of Pereira: Serenity and Accessibility
Pereira, nestled in Colombia’s Coffee Region, offers a serene and welcoming environment. This city is known for its natural beauty, temperate climate, and warm hospitality, which can significantly contribute to a patient’s overall well-being during their stay. The cost of living and medical care in Pereira can also present a more accessible option compared to the often prohibitive costs in the USA, without compromising on quality or safety standards.
The combination of advanced medical facilities, stringent regulatory oversight, a supportive patient journey framework, and a comforting environment makes Pereira a thoughtful consideration for those exploring options for ataxia, including investigational approaches focusing on cerebellar regeneration and motor control recovery. It’s about providing an environment where individuals can focus on their health journey with confidence and comprehensive support.
Your Guide: The Regencord Ataxia Journey Compass
To help navigate the complexities of considering investigational approaches for ataxia, we’ve developed the Regencord Ataxia Journey Compass. This branded patient resource tool is designed to provide clarity and empower informed decision-making:
- Self-Assessment Checklist: Understand your current symptoms, how ataxia impacts your daily life, and what you hope to achieve by exploring investigational pathways. This helps in framing your initial discussions.
- Information Gathering Guide: A structured approach to researching ataxia and regenerative medicine. This guide helps you identify reputable sources (like NIH, PubMed) and formulate questions for a confidential case review.
- Logistical Preparation Worksheet: For those considering international options, this tool helps you organize thoughts on travel, accommodation, support systems, and estimated timelines.
- Expectation Management Framework: Helps distinguish between realistic outcomes based on current scientific understanding and unverified claims. It encourages a focus on education and process rather than guaranteed results.
- Communication Prompts: A list of key questions to ask during your confidential case review to ensure all your concerns are addressed comprehensively.
The Regencord Ataxia Journey Compass is a practical resource to transform your uncertainty into a structured pathway towards informed action.
Our Regenerative Philosophy: An Educational and Patient-Centered Approach
At the core of the team at Regencord in Pereira, Colombia, is a deep commitment to an educational and patient-centered philosophy, especially when it comes to complex conditions like ataxia and the evolving field of regenerative medicine. Our approach is not about promising specific outcomes or offering unproven solutions, but about providing a clear, ethical, and supported pathway for individuals to explore potential options.
Focus on Education and Scientific Inquiry
We believe that true patient empowerment comes from knowledge. Our team emphasizes understanding the scientific basis of regenerative approaches, including the ongoing research into neural repair and cerebellar regeneration, rather than focusing on anecdotal evidence. We draw upon insights from leading scientific bodies and peer-reviewed literature, such as studies highlighted by the NIH and published in PubMed, to inform our communications.
The field of stem cell treatment for ataxia is an active area of investigation. Research published in PubMed-indexed journals explores various cell types and delivery methods, aiming to understand their potential for modulating disease progression or contributing to motor control recovery. These studies are critical for advancing our understanding of how these therapies might work and their safety profiles.
Source: U.S. National Institutes of Health (NIH), PubMed-indexed peer-reviewed journals, ClinicalTrials.gov.
The Role of Regenerative Medicine in Ataxia
Regenerative medicine aims to replace, engineer, or regenerate human cells, tissues, or organs to restore or establish normal function. In the context of ataxia, this field explores various avenues:
- Cellular Therapies: Investigating the potential of various cell types to support neural repair, reduce inflammation, or modulate the cellular environment in the cerebellum.
- Growth Factors and Biologics: Exploring compounds that might stimulate the body’s own regenerative processes.
- Gene Therapies: Researching ways to correct genetic defects responsible for certain hereditary ataxias.
It is crucial to understand that many of these approaches for ataxia are currently in research or investigational stages, and their long-term efficacy and safety are still being evaluated through rigorous clinical trials globally, as documented on platforms like ClinicalTrials.gov. Our philosophy is to openly discuss the current state of this research, without overstating its clinical application or promising definitive results.
Patient Empowerment Through Transparent Pathways
Our philosophy extends to ensuring that every individual who contacts us receives transparent information about the patient journey. This includes detailing the assessment process, potential investigational options that might be considered, the logistical support available, and the importance of post-treatment follow-up. We aim to create an environment where questions are welcomed, and decisions are made thoughtfully, based on comprehensive information and personal circumstances.
The team at Regencord in Pereira, Colombia, is committed to guiding you through this complex landscape with integrity, empathy, and a steadfast dedication to your well-being. We believe in providing the resources and support necessary to empower you to make the most informed decision possible for your health journey, always prioritizing education over persuasion and clarity over claims.
Overcoming Common Hesitations: Why Seeking Clarity is a Strategic Advantage

When considering investigational treatments for a condition like ataxia, especially in an international setting, it is natural to encounter a range of hesitations and concerns. These are valid questions that deserve clear, empathetic, and factual answers. Our team addresses these potential barriers by focusing on education and building trust.
Hesitation 1: “Are investigational stem cell treatments for ataxia legitimate and safe, especially abroad?”
Many patients wonder about the safety and legitimacy of novel treatments. The concern often stems from a lack of clear information. To address this, we emphasize the *research-driven* nature of regenerative medicine, distinguishing between unproven and *investigational* therapies. We highlight that reputable facilities adhere to strict protocols and operate within regulated frameworks. In Colombia, medical facilities offering advanced therapies are subject to the oversight of INVIMA and the Colombian Ministry of Health, which set standards for practice and product regulation. Our focus is on providing access to information regarding such investigational pathways, emphasizing patient safety and ethical considerations, rather than asserting unverified clinical superiority or guaranteeing outcomes. Our goal is to connect you with teams that uphold these standards, ensuring your peace of mind.
Hesitation 2: “This sounds too complicated and overwhelming. I wouldn’t know where to start with international medical care.”
The idea of coordinating international travel for medical care can seem daunting. The logistical anxiety is a significant barrier for many. We transform this perception by outlining a clear, step-by-step patient journey. From the initial confidential case review to travel arrangements, accommodation, and post-procedure support, our patient advocacy team provides comprehensive assistance. We present the entire process as a manageable, supported pathway, minimizing the burden on the patient and their family. This includes guidance on visa requirements, local transportation, and interpreting services, ensuring a smooth and supportive experience.
Hesitation 3: “What if it’s too expensive, or my insurance won’t cover it, making it inaccessible?”
Cost is a major concern for many considering advanced therapies. While many investigational treatments, particularly international ones, are often not covered by traditional insurance in countries like the USA, we believe in transparent discussions about the financial aspects. We frame the exploration of these options as an *investment* in one’s long-term health and quality of life. Without stating specific costs, we emphasize the comparative value of high-quality, comprehensive care in Colombia, which can often be more accessible than similar options in other parts of the world. Our focus is on ensuring patients understand the full scope of potential costs and value proposition before making a decision, empowering them with financial clarity.
Hesitation 4: “How do I know if I’m even a candidate for these types of approaches for ataxia, and what are the actual realistic expectations?”
Understanding candidacy and realistic expectations is paramount. We address this by emphasizing that the first and most critical step is always a thorough, confidential case review. This involves evaluating an individual’s specific medical history, type of ataxia, and overall health to determine if they might be a suitable candidate for existing investigational protocols or pathways. We are committed to fostering realistic expectations by openly discussing the current state of research, the goals of investigational therapies (e.g., neural repair, motor control recovery, symptom modulation), and the inherent uncertainties. This initial review is designed to provide clarity and an honest assessment, ensuring that any decision is based on a well-rounded understanding of potential benefits and limitations.
By directly addressing these common hesitations with transparency, education, and dedicated support, the team at Regencord in Pereira, Colombia, aims to build trust and empower individuals to explore all viable avenues for their ataxia journey with confidence.
Glossary of Key Terms
- Ataxia: A neurological disorder characterized by impaired coordination, affecting balance, speech, eye movements, and limb movements.
- Cerebellum: A major part of the brain located at the back of the skull, responsible for coordinating voluntary movements, balance, and motor learning.
- Regenerative Medicine: A branch of medicine that develops methods to regrow, repair, or replace damaged or diseased cells, organs, or tissues.
- Stem Cells: Undifferentiated biological cells that can differentiate into specialized cells and can divide to produce more stem cells. They are a focus of research in neural repair.
- Neural Repair: The process of repairing damaged components of the nervous system, often a target in neurological conditions like ataxia.
- Motor Control Recovery: The process of regaining or improving the ability to control voluntary movements, a key goal in ataxia management and research.
- Investigational Treatment: A treatment or therapy that is currently undergoing clinical trials or research to determine its safety and effectiveness, and is not yet widely approved for general use.
- INVIMA: Colombia’s National Food and Drug Surveillance Institute, responsible for regulating health products, similar to the FDA in the USA.
Frequently Asked Questions About Ataxia and Regenerative Approaches
What is ataxia, and how does it affect the body?
Ataxia is a neurological disorder that affects the part of the brain responsible for coordination, the cerebellum. This leads to problems with balance, walking, speech (dysarthria), swallowing (dysphagia), and fine motor tasks. It can manifest in various forms, including hereditary (e.g., Friedreich’s ataxia), sporadic, or acquired forms.
What is the current understanding of stem cell treatment for ataxia?
Research into ataxia stem cell treatment is an evolving field, focusing on various types of stem cells to investigate their potential in neural repair and cerebellar regeneration. The goal is to explore how these cells might help replace damaged neurons, reduce inflammation, or provide trophic support to existing cells, potentially aiding motor control recovery. These are primarily investigational approaches, with ongoing clinical trials worldwide documented by resources like ClinicalTrials.gov.
Why consider an international destination like Pereira, Colombia, for exploring advanced options?
Pereira, Colombia, offers several advantages for individuals exploring investigational regenerative approaches. It boasts a modern healthcare infrastructure, medical professionals with international training, and a supportive regulatory environment through INVIMA. Additionally, the region provides a serene environment conducive to healing, and the patient advocacy team at Regencord offers comprehensive logistical and educational support to simplify the patient journey.
Are there any guarantees of success with investigational regenerative treatments for ataxia?
It is crucial to understand that no medical treatment, particularly those in investigational stages, can guarantee specific outcomes or a “cure.” Our approach is centered on education, transparency, and a realistic understanding of potential benefits, which include supporting neural repair processes or modulating symptoms, rather than promising definitive results. The focus is on providing information about scientifically responsible pathways.
What kind of support can I expect from the Regencord team in Pereira?
The Regencord team provides comprehensive patient advocacy and support. This includes facilitating a confidential case review, assisting with travel and accommodation planning, coordinating appointments, providing language support, and offering ongoing educational resources. Our aim is to make the entire process as seamless and stress-free as possible, allowing you to focus on your health journey.
How do I determine if I am a suitable candidate for exploring regenerative pathways for ataxia?
The first step is a confidential case review. This involves a thorough evaluation of your medical history, diagnosis, current symptoms, and overall health status. This assessment helps the team understand your individual circumstances and determine if investigational regenerative pathways align with your needs and the current scientific understanding of ataxia and neural repair.
What should I expect during a confidential case review?
A confidential case review is an opportunity for you to share your medical records and discuss your condition and health goals with a dedicated patient advocate. This is a non-clinical consultation focused on education and evaluating the potential suitability of exploring available pathways. It is designed to answer your questions, address your concerns, and provide clear information without any obligation.
Empower Your Decision: Contact Us for a Confidential Case Review
The journey with ataxia can be challenging, but you don’t have to navigate the complex landscape of investigational treatments alone. The team at Regencord in Pereira, Colombia, is committed to providing clarity, education, and unwavering support as you explore all potential options for cerebellar regeneration and motor control recovery.
If you or a loved one are considering innovative approaches for ataxia stem cell treatment, we invite you to take the crucial first step towards an informed decision.
Discover if you are a candidate for the regenerative medicine pathways available through the team at Regencord in Pereira, Colombia. Contact us for a confidential case review.
Let us help you understand the possibilities and guide you through a patient journey focused on ethical care, transparent information, and comprehensive support. Your path to clarity begins here.