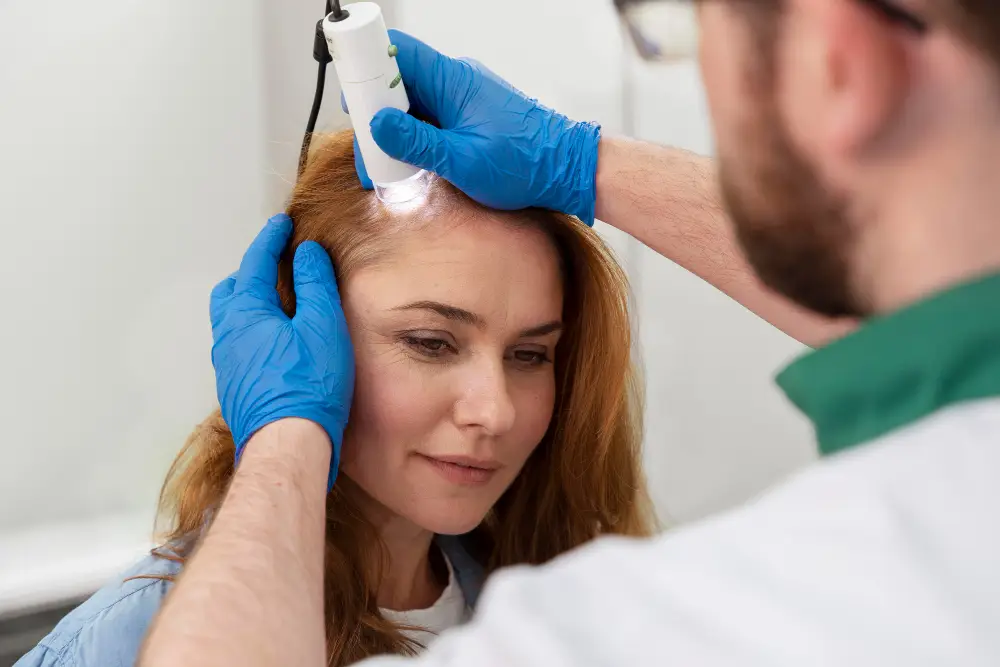
Placenta Stem Cell Treatment: Exploring Regenerative Pathways in Colombia
Key Takeaways
- Placenta-derived mesenchymal stem cells (MSCs) are being explored for their immunomodulatory and tissue regeneration properties in various research settings globally.
- Choosing to explore advanced therapies internationally, particularly in destinations like Pereira, Colombia, involves understanding both the science and the supportive patient journey.
- Colombia offers a regulated healthcare environment, with oversight from entities like INVIMA and the Ministry of Health, ensuring established standards for medical care.
- The team at Regencord in Pereira, Colombia, emphasizes an educational, patient-centric approach, focusing on providing clarity and support throughout the entire process.
- A confidential case review is a crucial first step to understand if regenerative medicine pathways align with your individual health needs and the current understanding of these evolving treatments.
Table of Contents
- Introduction: Navigating the Path to Clarity
- The Stakes: Understanding the Pursuit of Advanced Regenerative Pathways
- The Conventional Approach: Challenges in Established Healthcare Systems
- The Pereira, Colombia Advantage: A Context for Regenerative Exploration
- Introducing the “Regencord Pathway Clarifier”: Your Essential Resource
- Our Regenerative Philosophy: Education, Empowerment, and Individualized Review
- Overcoming Common Hesitations: Why Seeking Clarity is a Strategic Advantage
- Glossary of Key Terms
- Frequently Asked Questions (FAQ)
- Taking the Next Step: Your Confidential Case Review
- Disclaimer
Introduction: Navigating the Path to Clarity
The journey towards understanding and potentially accessing advanced regenerative therapies, such as those involving placenta stem cells, can feel complex and overwhelming. Many individuals facing persistent health challenges often seek options beyond conventional treatments, leading them to explore innovative pathways globally. This article aims to demystify the conversation around placenta stem cell treatment, focusing on mesenchymal stem cells (MSCs) and their documented properties related to immunomodulation and tissue regeneration, within the context of seeking care in Pereira, Colombia.
At Regencord in Pereira, Colombia, our team is dedicated to providing comprehensive, ethical, and patient-centric information. We believe that empowering you with well-researched insights, contextual understanding, and transparent guidance is the first step toward making informed decisions about your health. We understand the anxieties associated with exploring novel treatments and international travel for medical purposes. Our goal is to illuminate the patient journey, address common concerns, and introduce you to the advantages of considering Pereira as a destination for care, all while maintaining strict adherence to ethical and regulatory standards.
This resource is designed not just to inform but to guide you through the considerations that matter most, from the underlying science of fetal membrane therapy to the practicalities of international medical travel. We are here to help you move from uncertainty to decisive, informed action.
The Stakes: Understanding the Pursuit of Advanced Regenerative Pathways

For individuals living with chronic conditions or recovering from injuries, the search for effective therapies often involves navigating a landscape of hope and uncertainty. The human body’s inherent capacity for self-repair is a fascinating area of ongoing scientific inquiry. Regenerative medicine, including the exploration of stem cells, represents a frontier in this pursuit, aiming to support the body’s natural healing processes.
When conventional treatments yield limited results, patients often find themselves at a crossroads, compelled to explore all available avenues. This exploration is driven by a profound desire for improved quality of life, reduced discomfort, and greater functional capacity. The World Health Organization (WHO) continuously highlights the global burden of non-communicable diseases and musculoskeletal conditions, underscoring the universal need for effective and accessible therapeutic solutions. This global context fuels the interest in advanced approaches like placenta stem cell treatment, which leverages specific cellular properties for potential therapeutic benefit.
The implications of this search are deeply personal. Patients are not just seeking a therapy; they are seeking renewed hope, clarity, and a path forward that aligns with their values and health objectives. Understanding the scientific basis, ethical considerations, and logistical pathways for such treatments becomes paramount, particularly when considering international options.
(Reference to general WHO statements on global health burden of chronic diseases can be made here, without linking directly to placenta stem cells as a solution).
The Conventional Approach: Challenges in Established Healthcare Systems
Patients in many established healthcare systems, particularly in regions like North America or Europe, often encounter a series of challenges when seeking advanced or novel therapeutic options. These can include:
- Lengthy Waiting Lists: Access to medical consultations, diagnostic procedures, and even conventional treatments can involve significant delays, prolonging discomfort and anxiety.
- Limited Therapeutic Scope: Regulatory environments in some countries can be conservative, limiting access to emerging regenerative therapies that are still in investigational phases or used under different regulatory frameworks elsewhere.
- High Costs: For treatments not covered by public health systems or private insurance, the out-of-pocket expenses can be prohibitive, creating significant financial barriers.
- Fragmented Care: Navigating multiple specialists, administrative hurdles, and disconnected information systems can be emotionally and logistically draining.
These challenges can lead patients to feel that their options are constrained, prompting them to look internationally for solutions that align better with their health goals and timelines. It’s this drive for broader access and potentially more integrated care that often leads individuals to explore destinations with different healthcare paradigms, such as Colombia.
The Pereira, Colombia Advantage: A Context for Regenerative Exploration
For those considering advanced regenerative therapies, Pereira, Colombia, presents a compelling alternative, offering a unique blend of modern medical infrastructure, a supportive environment, and a patient-centric approach. Our experience reveals that a holistic approach, where patient advocates guide every step—from visa support and local accommodation to coordinating appointments and understanding cultural nuances—transforms a potentially daunting process into a seamless, reassuring experience. This integrated support system, inherent to the medical tourism infrastructure in Pereira, ensures patients can focus entirely on their well-being, rather than administrative burdens.
Robust Medical Infrastructure and Professional Acumen
Colombia has steadily built a reputation for its robust healthcare system, attracting international patients seeking quality medical services. Pereira, a vibrant city in the heart of Colombia’s Coffee Region, is no exception. It boasts modern medical facilities equipped with advanced diagnostic and therapeutic technologies. The medical professionals in Colombia are highly trained, with many having international education and experience, ensuring high standards of care.
This thriving medical landscape provides a strong foundation for innovative fields like regenerative medicine. Patients considering placenta stem cell treatment can find comfort in knowing they are exploring options within a healthcare system that values professional competence and modern medical practices.
Holistic Patient Journey Support
Navigating the landscape of advanced therapeutic options often means confronting a maze of regulations and cost barriers in one’s home country, pushing patients to seek high-quality, accessible alternatives abroad. Many are surprised to discover that destinations like Pereira, Colombia, have cultivated a robust ecosystem where innovative medical approaches, such as those leveraging mesenchymal stem cells, are integrated within a supportive, patient-centric framework designed to simplify the entire journey, from initial consultation to post-treatment care.
The Regencord team understands that the patient journey extends far beyond the clinical procedure itself. It encompasses logistical planning, cultural adaptation, and emotional support. In Pereira, a dedicated patient advocacy system is in place to assist with:
- Initial confidential case reviews and pathway clarification.
- Travel coordination, including airport transfers and local transportation.
- Accommodation arrangements that suit individual needs and budgets.
- Translation services to ensure clear communication with medical teams.
- Guidance on local amenities and cultural experiences to support a comfortable stay.
This comprehensive support network is designed to alleviate the stress of international medical travel, allowing patients and their companions to focus solely on the primary goal: health and recovery.
Ethical Oversight and Regulatory Frameworks
A crucial aspect of any medical journey, especially when considering novel therapies, is the assurance of ethical practice and regulatory oversight. Colombia’s Ministry of Health and Social Protection (Ministerio de Salud y Protección Social) and the National Food and Drug Surveillance Institute (INVIMA) are the primary governing entities responsible for regulating health services, medicines, and medical devices. These bodies work to ensure that medical procedures adhere to established national standards.
INVIMA, similar to the FDA in the United States, plays a vital role in supervising the quality and safety of health products and services. For regenerative medicine, this means that while research continues globally, any pathways offered within Colombia operate under a defined regulatory structure. This framework helps to build trust and ensure that patient safety and ethical considerations are paramount in the provision of advanced therapies. Patients can gain clarity by understanding that the Regencord team operates within these established guidelines, prioritizing transparency and informed decision-making.
(Reference to Colombian Ministry of Health and INVIMA for regulatory oversight, not specific approval of placenta stem cell therapies for all conditions).
Introducing the “Regencord Pathway Clarifier”: Your Essential Resource
To further support your journey in understanding and evaluating options for placenta stem cell treatment, the team at Regencord has developed a practical resource: the “Regencord Pathway Clarifier.” This branded tool is designed as a comprehensive checklist and guide to empower you with the right questions and considerations as you explore advanced regenerative medicine.
The “Regencord Pathway Clarifier” helps you:
- Understand the Fundamentals: Providing clear, accessible explanations of concepts like mesenchymal stem cells, immunomodulation, and tissue regeneration, drawing from reputable sources like the NIH and PubMed-indexed research.
- Navigate Your Options: Guiding you through critical questions to ask your medical team, both at home and when considering international care.
- Plan Logistically: Offering a step-by-step framework for preparing for international travel, including considerations for accommodation, transport, and communication.
- Set Realistic Expectations: Encouraging a balanced perspective by focusing on education and what to expect from a confidential case review, rather than unverified outcomes.
- Post-Treatment Considerations: Helping you think through follow-up care and integration of new insights into your long-term wellness plan.
This resource serves as a tangible companion, ensuring you have a structured approach to gathering information and making decisions confidently.
Our Regenerative Philosophy: Education, Empowerment, and Individualized Review
The Regencord team’s philosophy is rooted in education and patient empowerment. We recognize that the field of regenerative medicine is dynamic and requires a thoughtful, evidence-informed approach. We believe that true healing begins with clarity and a thorough understanding of the available pathways.
Our approach is centered on:
- Rigorous Information Sharing: Presenting information about placenta stem cell treatment, mesenchymal stem cells, and fetal membrane therapy based on current scientific understanding and ongoing research, referencing recognized institutions like the NIH.
- Individualized Case Review: Every patient’s journey is unique. We facilitate confidential case reviews where your medical history is thoroughly assessed to help determine if regenerative medicine pathways align with your specific situation. This is a critical step to manage expectations and ensure ethical guidance.
- Transparency and Trust: Maintaining open communication about processes, potential considerations, and the regulatory environment in Colombia. Our commitment is to build trust through honesty and comprehensive support.
- Patient Advocacy: Acting as your advocate, guiding you through the complexities of international medical travel and ensuring you feel supported at every stage.
We do not make claims of clinical superiority or guarantee outcomes. Instead, we focus on facilitating access to information and pathways that are explored within an ethical and regulated framework, empowering you to make the most informed choices for your health.
Overcoming Common Hesitations: Why Seeking Clarity is a Strategic Advantage
It’s natural to have questions and hesitations when considering advanced medical treatments, especially when they involve international travel. Our experience with patients exploring options for placenta stem cell treatment has highlighted several common concerns. Addressing these anxieties with clear, factual information is paramount to building trust and enabling confident decision-making.
Addressing Concerns about Legitimacy and Novel Therapies
Barrier: “Is placenta stem cell treatment legitimate or just a ‘fringe’ therapy, especially when sought internationally?”
Clarity & Trust: The scientific understanding of placenta-derived mesenchymal stem cells (MSCs) and their potential for immunomodulation and tissue regeneration is an active area of global research, as highlighted by various studies indexed in PubMed and supported by institutions like the NIH. While research is ongoing, the therapeutic potential of MSCs is widely recognized. At Regencord, the team focuses on pathways supported by ethical considerations and evolving scientific understanding. We operate within Colombia’s robust regulatory frameworks, overseen by bodies like INVIMA and the Ministry of Health, which ensure patient safety and ethical practices. Our approach is grounded in facilitating access to thoroughly reviewed information, rather than making unverified claims about specific outcomes.
Ensuring Trust in International Medical Care Quality
Barrier: “How can I trust the quality and safety of medical care in a country like Colombia?”
Clarity & Trust: Colombia has a well-developed healthcare infrastructure and a strong reputation for medical innovation within Latin America. Its medical professionals undergo rigorous training, often with international experience, ensuring high standards of care. Institutions like INVIMA and the Colombian Ministry of Health are instrumental in establishing and enforcing national health policies and standards, including those for advanced therapies. Pereira, in particular, has cultivated a reputation as a medical tourism destination due to its modern facilities and a dedicated focus on international patient care standards. By choosing Regencord, you are choosing a pathway that emphasizes transparency regarding these national regulatory bodies and aligns with global ethical considerations, moving your focus from fear of the unknown to an understanding of a well-regulated system.
Simplifying the Complexity of International Medical Travel
Barrier: “The logistics of international medical travel for something as complex as stem cell treatment seem overwhelming and complicated.”
Clarity & Trust: We understand that coordinating international medical travel can appear daunting. This is precisely why the Regencord team offers comprehensive patient advocacy and support. From your initial confidential case review, we provide clear, step-by-step guidance on every aspect of your journey. This includes assistance with travel arrangements, local accommodation, understanding local customs, and ensuring seamless communication with medical professionals. Our role is to transform a potentially complex process into a manageable, supported pathway, allowing you to focus on your well-being. The “Regencord Pathway Clarifier” tool is also designed to help you organize your thoughts and preparations, providing a structured approach to your journey.
Navigating Expectations: Is This Right for My Condition?
Barrier: “Will this treatment actually work for *my* condition? I don’t want false hope.”
Clarity & Trust: We emphasize realistic expectations and individualized assessment above all else. The decision to explore placenta stem cell treatment is deeply personal and must be based on a thorough understanding of your unique medical situation and the current scientific landscape. The Regencord team facilitates a confidential case review, where your specific health history, diagnostic information, and desired outcomes are carefully evaluated by the medical team. This process is designed to determine if you are a potential candidate for the regenerative medicine pathways offered, ensuring that any discussions about potential approaches are grounded in a careful evaluation of scientific understanding and ethical considerations, rather than generalized promises. Our focus is on providing clarity and managing expectations realistically, respecting your autonomy in making informed health decisions.
Glossary of Key Terms
- Placenta Stem Cell Treatment: Refers to therapeutic approaches utilizing stem cells derived from placental tissues, often focusing on their potential regenerative and immunomodulatory properties.
- Mesenchymal Stem Cells (MSCs): Multipotent stromal cells that can differentiate into various cell types (e.g., bone, cartilage, fat) and possess significant immunomodulatory and anti-inflammatory properties. Placenta is a rich source of MSCs.
- Immunomodulation: The process by which a substance or treatment modifies the immune response, either by suppressing or enhancing it. MSCs are studied for their ability to influence immune system activity.
- Tissue Regeneration: The process of replacing or repairing damaged tissues and organs through the stimulation of new tissue growth. This is a primary area of interest for regenerative medicine, including stem cell therapies.
- Fetal Membrane Therapy: Refers to therapeutic applications involving components of the fetal membranes (amnion and chorion), which are sources of various bioactive factors and cells, including MSCs, known for their healing properties.
- INVIMA: Colombia’s National Food and Drug Surveillance Institute (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), a regulatory body responsible for inspecting and supervising the production and marketing of health products and services.
- Patient Advocate: An individual or team dedicated to assisting patients in navigating the healthcare system, providing support, information, and coordination of services, especially for international medical travelers.
Frequently Asked Questions (FAQ)
What are placenta stem cells and how are they used?
Placenta stem cells, particularly mesenchymal stem cells (MSCs) derived from the placenta and fetal membranes, are multipotent cells studied for their potential to modulate immune responses, reduce inflammation, and support tissue regeneration. Research indicates they secrete various growth factors and cytokines that can contribute to cellular repair and healing processes. Their use is being explored in a wide range of conditions, and therapies generally involve introducing these cells into the body to support regenerative pathways. Always consult with medical professionals to understand the specific applications and current understanding.
Why consider placenta stem cell treatment in Pereira, Colombia?
Pereira, Colombia, offers a modern healthcare environment with well-trained medical professionals and state-of-the-art facilities. Colombia’s regulatory bodies, such as INVIMA and the Ministry of Health, oversee medical practices, ensuring compliance with national standards. For international patients, Pereira provides a supportive ecosystem for medical tourism, including comprehensive patient advocacy services that help with travel logistics, accommodation, and communication. This combination offers an accessible and supportive pathway for exploring advanced regenerative options.
Is placenta stem cell treatment legally regulated in Colombia?
Yes, medical practices in Colombia, including those involving regenerative medicine, operate under the oversight of the Colombian Ministry of Health and Social Protection and INVIMA. These institutions establish guidelines and standards to ensure ethical practice and patient safety. Any treatments offered through the team at Regencord in Pereira adhere to these national regulations, focusing on ethical considerations and informed consent.
What is the typical patient journey like when seeking care through Regencord?
The journey begins with a confidential case review, where our team and associated medical professionals evaluate your medical history and assess your candidacy for available pathways. If you are deemed a potential candidate, we then provide detailed information about the process, logistical support for travel to Pereira (accommodation, transport, translation), and on-site assistance. Post-treatment, we remain a resource for follow-up guidance. Our aim is to make the entire process as clear and stress-free as possible.
How do I know if placenta stem cell treatment is right for me?
Determining if placenta stem cell treatment is a suitable option for your specific condition requires a thorough, individualized medical assessment. This is why we emphasize starting with a confidential case review. The medical team will review your diagnostic tests, medical history, and current health status to discuss whether regenerative medicine pathways align with your needs and the current scientific understanding. We do not promote blanket solutions; our focus is on personalized evaluation and education.
Discover if you are a candidate for the regenerative medicine pathways available through the team at Regencord in Pereira, Colombia. Contact us for a confidential case review.