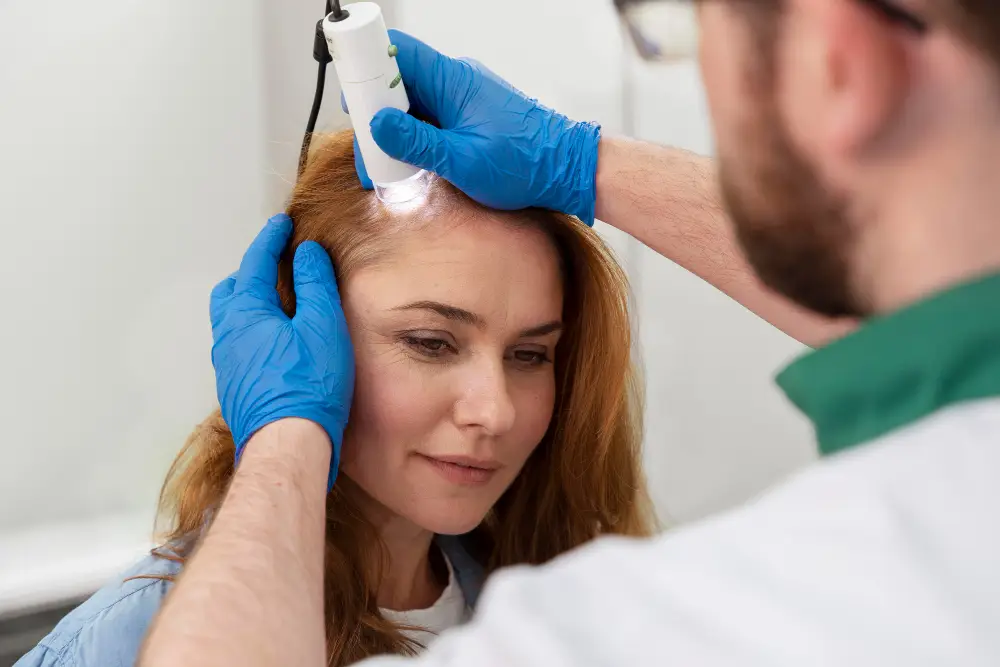
Exploring Stem Cell Vision Pathways: A Comprehensive Guide to Regenerative Approaches
Key Takeaways
- Stem cell research for vision is a rapidly evolving field, holding significant promise but largely remaining investigational for many conditions.
- Responsible exploration of regenerative medicine pathways requires understanding the science, regulatory frameworks, and ethical considerations.
- Many conventional treatments for conditions like macular degeneration or optic nerve damage address symptoms but may not regenerate tissue.
- Colombia, particularly cities like Pereira, offers a well-regulated healthcare system with modern facilities for exploring ethical regenerative medicine pathways.
- A dedicated patient advocacy team can simplify the complex logistics of seeking advanced medical care internationally, focusing on transparency and support.
- The Regencord team in Pereira, Colombia, is committed to patient education and facilitating access to rigorously reviewed, ethically grounded regenerative medicine options for vision challenges.
Introduction: Navigating Hope and Reality in Vision Health
For individuals facing the challenges of vision impairment, the journey is often marked by a blend of profound hope and deep uncertainty. Conditions affecting the retina, macula, or optic nerve can profoundly impact daily life, leading many to seek out the most advanced and innovative options available. Among these, the concept of “stem cell vision treatment” and broader regenerative medicine approaches often emerges as a beacon of potential for retinal regeneration, macular degeneration, vision restoration, and optic nerve repair.
At Regencord in Pereira, Colombia, we understand the complexities and emotional weight of this search. Our role is to provide clear, ethical, and evidence-informed guidance for those exploring regenerative pathways. This comprehensive guide aims to demystify the current landscape of regenerative medicine as it relates to vision, helping you understand what is known, what is investigational, and how a responsible approach can be navigated within a well-regulated international setting.
We believe that informed decision-making is the cornerstone of effective patient advocacy. This resource is designed to empower you with knowledge, addressing common concerns and outlining the supportive framework provided by our team. We focus on education, transparency, and a patient-centric journey, ensuring that your exploration of advanced vision care is as clear and supported as possible.
The Stakes: Critical Implications of Vision Challenges

Vision is fundamental to our interaction with the world, and any threat to it can be deeply distressing. Conditions such as age-related macular degeneration, diabetic retinopathy, glaucoma-related optic nerve damage, or inherited retinal dystrophies can lead to irreversible vision loss. The impact extends far beyond sight, affecting independence, mental well-being, and overall quality of life.
According to the World Health Organization (WHO), hundreds of millions of people worldwide live with vision impairment, with a significant proportion suffering from conditions that currently have no comprehensive regenerative solution. The urgency to find effective pathways for retinal regeneration, macular degeneration, vision restoration, and optic nerve repair is therefore immense.
The National Eye Institute (NEI), a part of the U.S. National Institutes of Health (NIH), has highlighted the extensive research being conducted into regenerative approaches for ocular diseases. This research, often detailed in PubMed-indexed peer-reviewed journals and tracked on ClinicalTrials.gov, explores how cellular therapies might one day help replace damaged cells, protect existing ones, or create a more supportive environment for visual function. However, the NEI also consistently stresses that many of these approaches are still in experimental stages, requiring careful and ethical evaluation.
Patients often feel a profound sense of urgency, especially when faced with progressive conditions. This urgency can sometimes lead to seeking out treatments that lack robust scientific backing or operate outside established ethical and regulatory oversight. Our firm belief, honed from years of facilitating international medical care, is that navigating this landscape requires a meticulous commitment to verifiable facts and a clear understanding of the scientific and regulatory context. The choice to explore advanced care is a deeply personal one, but it should always be an informed one, grounded in a partnership with a team that prioritizes your safety and well-being above all else.
The Conventional Approach in the USA: Limitations and Current Standards

In countries like the USA, the standard of care for many vision-threatening conditions is highly advanced, focusing on managing disease progression, preserving existing vision, and treating symptoms. For conditions like age-related macular degeneration (AMD), treatments might involve anti-VEGF injections to slow progression in wet AMD, or lifestyle modifications and nutritional supplements for dry AMD. Glaucoma is typically managed with eye drops, laser treatments, or surgery to reduce intraocular pressure and protect the optic nerve from further damage.
These conventional approaches, regulated by bodies like the U.S. Food and Drug Administration (FDA), are effective in many cases for slowing the decline of vision or managing specific symptoms. However, they often do not offer a path to true retinal regeneration or optic nerve repair. Once significant cellular damage has occurred, the body’s natural regenerative capacity for ocular tissues is limited, and current conventional therapies rarely restore lost vision.
This limitation is a significant driver for individuals to explore investigational therapies, including those involving stem cells. While research into stem cell applications for vision is robust in the USA, largely supported by the NIH and undergoing rigorous clinical trials registered on ClinicalTrials.gov, access to these investigational treatments outside of a clinical trial setting is highly restricted. The FDA maintains strict regulations on unproven therapies, emphasizing the need for extensive research and demonstrated efficacy and safety before widespread clinical application. This creates a gap for patients who have exhausted conventional options but may not qualify for or have access to ongoing clinical trials.
Understanding these limitations is crucial. It clarifies why patients begin to look beyond their immediate geographic boundaries, seeking environments where ethical, investigational regenerative medicine pathways might be more accessible, always within a framework of rigorous oversight and patient safety.
The Pereira, Colombia Advantage: A New Lens for Regenerative Medicine
When considering advanced medical pathways, especially those involving regenerative medicine for vision challenges, the choice of location extends beyond the clinical setting. It encompasses regulatory integrity, the patient experience, and the overall environment. Pereira, Colombia, offers a compelling confluence of these factors, providing a distinct advantage for those exploring new possibilities.
Our deep experience in facilitating international medical care has revealed that patients navigating complex conditions often face not just a medical challenge, but an emotional and logistical labyrinth. Simplifying this pathway transforms perceived complexity into a manageable, empowering journey. Beyond the clinic, the recovery environment significantly impacts well-being, and Pereira offers a unique blend of high-standard medical facilities within a serene, supportive cultural setting, which we’ve seen aids patient recovery and reduces stress.
Colombia’s Robust Healthcare & Regulatory Standards
Colombia has earned a reputation for its advanced healthcare system, particularly in major cities. The country’s commitment to high medical standards is overseen by the Colombian Ministry of Health and the National Food and Drug Surveillance Institute (INVIMA). INVIMA plays a role similar to the FDA, meticulously regulating medical products, procedures, and facilities to ensure safety and quality. This robust oversight means that facilities offering regenerative medicine pathways adhere to strict protocols, providing a secure and reliable environment for patients.
Medical professionals in Colombia undergo rigorous training, often with international experience, ensuring that care is delivered with skill and precision. Modern medical infrastructure, including state-of-the-art diagnostic equipment and treatment facilities, is readily available. For sensitive procedures related to vision care, this level of infrastructure and regulatory diligence is paramount, instilling confidence that patients are engaging with a system that prioritizes their well-being.
A Holistic Patient Journey: Beyond Clinical Care
The decision to explore advanced medical pathways abroad is a deeply personal one, driven by both hope and practical considerations. We’ve observed that the unique blend of a modern, well-regulated healthcare infrastructure with a culturally rich, serene environment—like that found in Pereira—provides a powerful synergy. This combination not only facilitates access to cutting-edge investigational approaches under strict ethical guidelines but also creates a restorative atmosphere that reduces stress and supports emotional well-being, which is often overlooked but critical in serious medical journeys.
The team at Regencord in Pereira, Colombia, understands that the patient journey extends far beyond the clinic walls. For individuals seeking options for retinal regeneration, macular degeneration, vision restoration, or optic nerve repair, the process can be daunting. Our approach focuses on a holistic support system:
- Personalized Case Review: A confidential, in-depth evaluation of your specific medical history and vision challenges to determine potential candidacy for available regenerative medicine pathways.
- Logistical Facilitation: Assistance with travel arrangements, accommodation, local transportation, and navigating necessary documentation, making the international journey seamless.
- Cultural Integration: Support in adjusting to the local environment, including language assistance and guidance on local customs, ensuring comfort and peace of mind.
- Ongoing Advocacy: A dedicated advocate to answer questions, coordinate appointments, and provide continuous support throughout your time in Pereira.
This comprehensive support system aims to alleviate the non-clinical burdens, allowing patients to focus entirely on their health and potential regenerative pathways.
Cultural Comfort and Serene Environment
Pereira, nestled in Colombia’s picturesque Coffee Region, offers a uniquely calming and supportive backdrop for medical travel. Its moderate climate, lush landscapes, and warm, welcoming culture provide an environment conducive to recovery and peace of mind. The pace of life, while vibrant, allows for tranquility, a stark contrast to the often-stressful environments associated with urgent medical decisions.
The local cuisine, friendly people, and opportunities for gentle recreation contribute to a sense of well-being that complements any medical journey. This blend of high-quality medical infrastructure within a restorative setting is a key component of the Regencord patient experience, distinguishing it from conventional medical travel and underscoring our commitment to the patient’s holistic health.
The Vision Clarity Pathway Checklist: Your Resource for Informed Decisions
Navigating the complex world of regenerative medicine for vision can feel overwhelming. To empower you with clarity and structure, the Regencord team has developed The Vision Clarity Pathway Checklist. This practical tool is designed to help you systematically evaluate your options, understand the process, and prepare for a confidential case review.
What The Vision Clarity Pathway Checklist Helps You Do:
- Assess Your Current Situation: A structured way to document your specific vision condition, its progression, and prior treatments, ensuring you have all relevant medical history ready for review.
- Understand Regenerative Concepts: Provides key questions to consider regarding the science of retinal regeneration, macular degeneration, vision restoration, and optic nerve repair, helping you differentiate between established research and early-stage investigations.
- Evaluate Ethical Considerations: Guides you through important ethical questions to ask about any potential pathway, reinforcing the need for transparent, responsible care.
- Prepare for International Travel: Outlines key logistical considerations, from documentation to accommodation, so you know what to expect and what to prepare for if you consider care in Pereira, Colombia.
- Formulate Key Questions: Helps you articulate your most pressing questions for the Regencord team, ensuring you get the information you need to make confident decisions.
- Track Your Journey: A practical framework to track steps from initial inquiry to post-review considerations, bringing order to a potentially complex process.
This checklist is not a diagnostic tool, nor does it guarantee outcomes. Instead, it serves as your personal guide to becoming a more informed and empowered participant in your health journey. It reflects our commitment to patient education and transparent communication, ensuring you feel supported and prepared at every stage of exploring regenerative pathways for your vision.
Contact us to learn more about how to utilize The Vision Clarity Pathway Checklist in preparation for your confidential case review with the Regencord team.
Our Regenerative Philosophy and Approach: Education and Ethical Pathways
At Regencord in Pereira, Colombia, our philosophy is rooted in responsible innovation, ethical practice, and comprehensive patient education. We recognize that the term “stem cell vision treatment” can evoke both immense hope and significant caution, given the spectrum of information available, from rigorously researched science to speculative claims.
Our approach is not to make unverified clinical assertions or offer generic “treatments.” Instead, we focus on facilitating access to regenerative medicine pathways that are grounded in scientific principles, adhere to stringent ethical guidelines, and operate within the clear regulatory framework established by the Colombian Ministry of Health and INVIMA. This means:
- Focus on Investigational Approaches: We help patients explore options that, while potentially transformative, are often considered investigational. We provide transparent information about the current state of research into retinal regeneration, macular degeneration, vision restoration, and optic nerve repair, as often highlighted by sources like the NIH and PubMed-indexed journals.
- Ethical Scrutiny: Every regenerative medicine pathway facilitated through Regencord undergoes rigorous ethical review. We prioritize patient safety, informed consent, and a clear understanding of potential benefits and inherent limitations. Our goal is to connect patients with care that exemplifies the highest standards of medical ethics.
- Personalized Assessment: We believe that no two patient journeys are alike. Our process begins with a confidential, thorough review of your unique medical situation. This allows us to determine if you are a potential candidate for the specific regenerative medicine pathways available, ensuring that any exploration is highly individualized and appropriate for your condition.
- Patient Empowerment Through Knowledge: We are advocates for empowering patients with accurate, accessible information. Our team helps you understand the underlying science, the methodology of specific regenerative approaches, and the expected patient journey, dispelling myths and fostering realistic expectations.
- Collaboration with Reputable Facilities: We partner with medical facilities in Pereira that uphold the highest standards of care, equipped with modern technology and staffed by dedicated medical professionals experienced in advanced cellular therapies.
Our commitment is to guide you through this complex landscape with integrity, offering clarity and support as you consider the potential of regenerative medicine for your vision health. We aim to be your trusted resource, helping you navigate responsible pathways with confidence and peace of mind.
Overcoming Common Hesitations: Why Seeking Clarity is a Strategic Advantage
Considering advanced care, especially abroad, naturally comes with questions and concerns. The Regencord team understands these hesitations and addresses them proactively, turning potential barriers into opportunities for clarity and confidence. Based on our extensive experience and insights into the patient journey, we’ve identified key areas of concern and how our approach directly addresses them.
“Is Regenerative Vision Care Legitimate or Just Hype?”
Many patients express a valid skepticism, having encountered sensationalized claims about “stem cell vision treatment.” This concern is legitimate, as the field has seen both remarkable scientific progress and unfortunately, instances of unregulated, unproven therapies.
Our Approach: We address this by emphasizing Regencord’s unwavering commitment to ethical, evidence-informed exploration. While much in vision-related stem cell therapy is investigational, we operate strictly within stringent Colombian regulatory frameworks (INVIMA, Ministry of Health) for the *responsible application* of regenerative principles. Our focus is on patient education about the current state of science, adherence to ethical guidelines, and facilitating access to pathways that are rigorously reviewed. We prioritize transparency about what is known, unknown, and what constitutes a responsible approach to advanced cellular therapies, ensuring we never make unsubstantiated claims. Our goal is to connect you with care that aligns with robust scientific inquiry and regulatory oversight.
“What About Medical Quality and Safety in Colombia?”
Patients from the USA and other developed nations often worry about the quality and safety of international medical care, especially for sensitive conditions like vision loss.
Our Approach: We reframe this perception by focusing on verifiable facts about Colombia’s healthcare system. Colombia boasts a robust, internationally recognized healthcare infrastructure. We highlight modern, accredited facilities in Pereira and the high standards of medical training for its professionals. INVIMA’s rigorous oversight of medical products and procedures ensures quality and safety, aligning Colombian standards with international best practices. Regencord connects patients with facilities that uphold these exacting levels of quality and safety, providing peace of mind through documented standards and a commitment to patient welfare.
“International Travel for Vision Care Seems Overwhelming.”
The thought of coordinating international travel, medical records, appointments, and accommodation while managing a serious health condition can be daunting.
Our Approach: We directly address logistical anxiety by outlining a clear, supportive, step-by-step patient journey. The Regencord patient advocacy team provides comprehensive support: from an initial confidential review and assistance with medical records transfer, to travel logistics, local accommodations, and cultural integration. This transforms the perceived complexity into a manageable, guided pathway. Our message is clear: the patient never navigates this journey alone; our dedicated team is there at every step to ensure a smooth and comfortable experience.
“I’m Concerned About Costs and Hidden Fees.”
Financial considerations are always a significant factor, and many fear hidden costs or unclear pricing when considering international medical care.
Our Approach: We emphasize transparency and value. While avoiding specific price quotes in public forums, we explain that Regencord focuses on providing a clear understanding of the *scope* of services and *pathway options* during a confidential review. Patients often find a compelling value proposition in Colombia, combining high-quality care and supportive infrastructure with a potentially more accessible cost structure compared to other regions. Our commitment is to ensure that all financial decisions can be made with full clarity regarding the services provided by the Regencord team, fostering trust through open communication about the value and components of the patient journey.
Glossary of Key Terms
- Regenerative Medicine: A broad field focused on developing therapies that repair, replace, or regenerate damaged or diseased cells, tissues, or organs to restore normal function.
- Stem Cells: Undifferentiated biological cells that can differentiate into specialized cells and can divide to produce more stem cells. They are fundamental to the body’s repair processes.
- Retinal Regeneration: The process of repairing or replacing damaged cells within the retina, the light-sensitive tissue at the back of the eye essential for vision.
- Macular Degeneration: A common age-related eye condition that damages the macula, the central part of the retina responsible for sharp, detailed central vision.
- Vision Restoration: The process of recovering lost visual function, either partially or fully, through medical intervention.
- Optic Nerve Repair: Efforts to heal or regenerate the optic nerve, which transmits visual information from the eye to the brain, when it has been damaged.
- Investigational Pathway: A medical approach or therapy that is still under scientific study and evaluation, not yet fully approved or considered standard of care.
- FTC Compliance: Adherence to regulations set by the U.S. Federal Trade Commission, ensuring advertising and marketing are truthful and not deceptive.
- INVIMA: Colombia’s National Food and Drug Surveillance Institute, the regulatory body responsible for overseeing the safety and quality of medical products and procedures.
- Patient Advocacy: The act of supporting and representing the best interests of a patient, ensuring their needs are met and their rights are protected throughout their medical journey.
Frequently Asked Questions About Regenerative Vision Pathways
- What specific vision conditions might be explored through regenerative pathways?
- Regenerative medicine research is actively exploring potential applications for a range of conditions, including certain forms of macular degeneration, retinal dystrophies, optic nerve damage, and other degenerative eye conditions. It’s crucial to understand that many of these are still within investigational frameworks. A confidential case review with the Regencord team can help determine if your specific condition aligns with available pathways.
- Is “stem cell vision treatment” recognized as a standard therapy?
- For many vision conditions, direct “stem cell vision treatment” that offers vision restoration or retinal regeneration is still largely in the investigational phase and is not yet a standard, widely approved therapy in most parts of the world. Reputable research, often cited by the NIH and published in peer-reviewed journals, continues to explore its potential, but caution is advised regarding unproven claims. The Regencord team focuses on facilitating access to ethical pathways that adhere to strict regulatory guidelines.
- How does Regencord ensure the ethical nature of these pathways?
- Our commitment to ethics is paramount. We work with facilities that operate under the rigorous oversight of the Colombian Ministry of Health and INVIMA. All pathways are subject to careful ethical review, ensuring informed consent, transparent communication about potential benefits and limitations, and strict adherence to patient safety protocols. Our philosophy prioritizes responsible science over unverified promises.
- What should I expect during a confidential case review?
- During a confidential case review, you will share your comprehensive medical history, diagnostic reports, and details of your vision challenges with the Regencord team. This allows our patient advocates to conduct a thorough preliminary assessment, discuss the current landscape of regenerative medicine relevant to your condition, and determine if you are a potential candidate for the pathways we facilitate. It’s an opportunity for you to ask questions and gain clarity on your options without obligation.
- What are the potential risks associated with exploring investigational regenerative pathways for vision?
- As with any medical intervention, especially investigational ones, there can be potential considerations. These vary depending on the specific pathway. The Regencord team and the medical providers we connect you with prioritize a comprehensive discussion of all known factors, helping you understand any potential considerations associated with your individual case and chosen pathway. This is a critical component of informed consent and patient safety.
- How long does the entire process, from inquiry to potential care, typically take?
- The timeline can vary significantly based on individual medical needs, the complexity of the case, and travel logistics. From your initial confidential case review to the completion of a potential pathway in Pereira, the Regencord team works diligently to streamline the process. We will provide a more personalized estimate after your initial review, ensuring transparent communication at every stage.
Your Next Step Towards Clarity
Navigating complex vision challenges and the evolving landscape of regenerative medicine requires trusted guidance. The Regencord team in Pereira, Colombia, is dedicated to providing that clarity, education, and unwavering support.
If you are exploring advanced options for retinal regeneration, macular degeneration, vision restoration, or optic nerve repair, and seek a responsible, ethical pathway within a supportive environment, we invite you to take the first confident step.
Discover if you are a candidate for the regenerative medicine pathways available through the team at Regencord in Pereira, Colombia. Contact us for a confidential case review.